Description
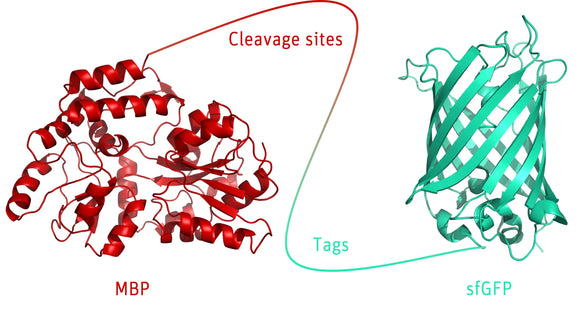
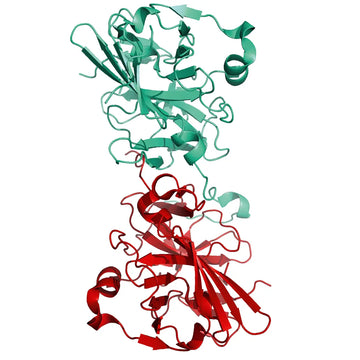
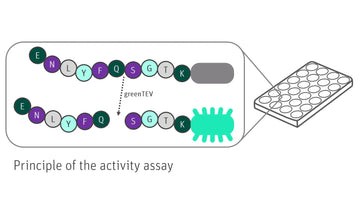
The Cleavage and Tag Control Protein is a versatile tool consisting of various elements and cleavage sites for different proteases. Furthermore, it's equipped with multiple tags to serve as positive control for Western Blot analyses or functional assays.
The Cleavage and Tag Control Protein is available in liquid or lyophilized form. The format and size can be selected by using the buttons.
-
Product Name: Cleavage and Tag Control Protein
-
Catalog No.: P2020-131 liquid, P2020-141 lyophilized
-
Synonyms: control protein, reference protein, cleavage control protein, multiple tag control protein, universal reference protein