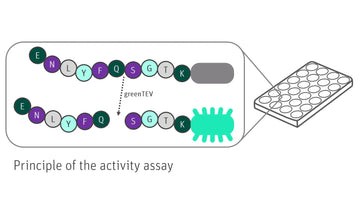
greenTEV represents the catalytic domain of the nuclear inclusion a (NIa) protein with a molecular weight of 27 kDa encoded by the plant virus Tobacco Etch Virus. "green" indicates fusion of the protease to green fluorescent protein (GFP), which leads to increased stability and solubility of TEV protease. Moreover, greenTEV has been optimized by site directed mutagenesis to prevent autocatalytic cleavage. greenTEV is a highly site-specific cysteine protease that recognizes the amino acid sequence Glu-Asn-Leu-Tyr-Phe-Gln-(Gly/Ser) [ENLYFQ(G/S)] and cleaves between the residues Gln and Gly/Ser. The most commonly used recognition sequence is ENLYFQG. In biotechnology, greenTEV is a versatile enzyme to remove affinity tags from recombinant proteins with high specificity and activity over a wide range of pH, ionic strength and temperatures between 4 °C and 30 °C. The optimal temperature for cleavage is 30 °C. It is recommended to improve cleavage efficiency for each fusion protein by varying the amount of recombinant greenTEV, reaction time, or incubation temperature. The great advantage of greenTEV is its facile removal after cleavage reaction by immobilized metal affinity chromatography (IMAC) since it is equipped with a His-tag. Furthermore, the removal of greenTEV can be monitored instantly by detection of fluorescence in solution - this easy, fast and sensitive method omits time-consuming SDS-PAGE or Western blot analysis.
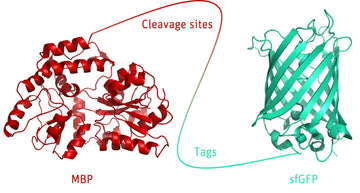
The Cleavage and tag control protein is a versatile protein consisting of various cleavage sites for proteases including TEV, Enterokinase, Thrombin, Factor Xa and PreScission™. Protease cleavage results in generation of two or three fragments that have significantly reduced molecular weights, which are as follows:
- TEV cleavage: 42.8 kDa and 47.7 kDa
- Enterokinase: 43.5 kDa, 20.1 kDa and 26.9 kDa
- Thrombin: 43.9 kDa and 46.6 kDa
- Factor Xa: 44.3 kDa and 46.2 kDa
- PreScission™: 45.3 kDa and 45.3 kDa
Moreover, the 90.5 kDa fusion protein is equipped with multiple tags such as Twin-Strep-, HA-, FLAG-, and His-tag, for instance, that may serve as positive control performing specific Western blot analyses or any functional assay. Since the protein is fused to GFP, it is additionally applicable as fluorescence control. Thus, the cleavage and tag control protein provides an excellent tool to monitor protease cleavage reactions and specific Western blot analyses as well as fluorescence experiments.
Additional information greenTEV
|
SDS-PAGE/Coll. Coomassie
|
Histogram of marked lane in gel picture
|
 |
|
|
Test Cleavage
|
Description of Test Cleavage
|
|
|
|
Additional information for Cleavage and tag control protein
|
SDS-PAGE/Coll. Coomassie
|
Histogram of marked lane in gel picture
|
|
|
 |
|
Test Cleavage
|
Description of Test Cleavage
|
|
|
|
|
Test Cleavage
|
Description for Test Cleavage
|
|
|
|